Overview
ISO 15189, the FSR Code and ILAC G19 are consistent in the requirement that consumables that can affect the quality of examination shall be verified for performance before use in examinations.
To fulfil this requirement the FCN, in collaboration with Cellmark Forensic Services and SceneSafe, conducted a National Consumable Validation. Every SARC in England and Wales (55 SARCs in total) participated in this validation, with each providing 5 forensic DNA grade consumables from their consumable stores which were then tested for presence of contaminating DNA. 19 different consumable types were tested from 114 different batches and the consumables tested were manufactured and supplied to end users up to ~2 years prior to testing. In total 261 forensic DNA grade consumables were tested and every one of these consumables passed testing, as per the acceptance criteria for DNA consumables defined in FSR-C-108.
In addition, 14 non forensic DNA grade consumables were tested and 6 of these items failed, whereby unacceptable levels of DNA were identified on these consumables, further demonstrating the importance of SARCs using forensic DNA grade consumables for the recovery of forensic evidence
The data from this National Consumable Validation demonstrates that these consumables have been manufactured to the required forensic DNA grade standard and their integrity has been maintained up to the point of use by all the SARCs in England and Wales.
How it worked
The national consumable validation process started off with identifying a national demand for support from the FCN to validate SARC consumables. After exploring options on how the FCN can support SARCs with this work we quickly identified this as a perfect opportunity for a centralised validation. After a scoping exercise for engagement in this project the number of SARCs participating continued to increase until we had every single SARC in England and Wales signed up to participate in this central validation.
The FCN worked in collaboration with SceneSafe and Cellmark Forensic Services to develop an effective and robust national consumable validation plan.
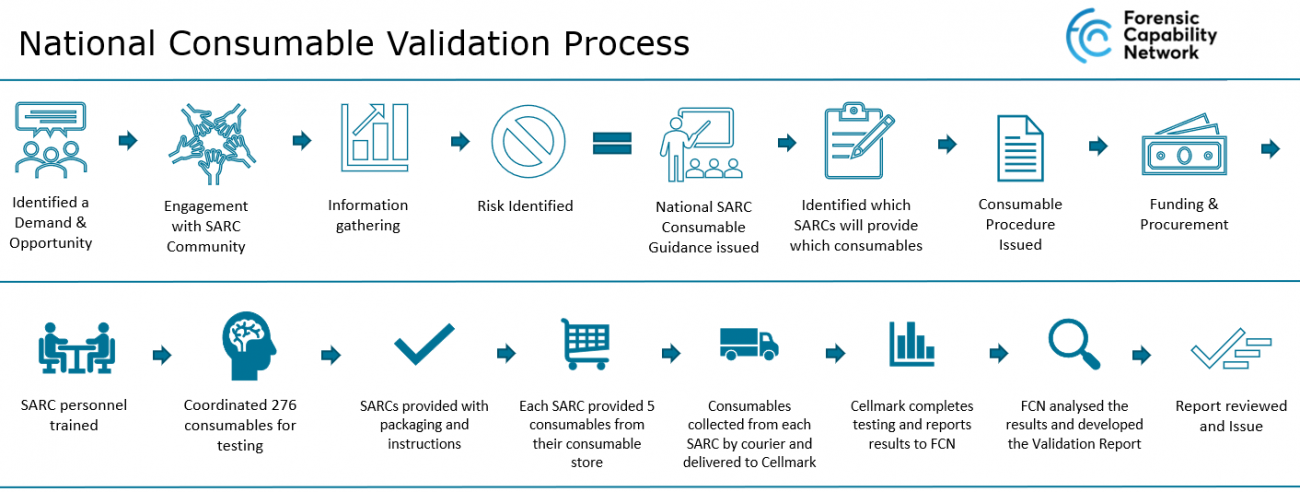
To gain a better understanding of the SARC consumable receipt, storage and handling process and to gather information on which consumables SARC’s use, a survey was issued to the SARCs. The results from this survey highlighted some key risks:
After implementing resolutions to the risks identified, SARCs nationally started working to consumable receipt, storage and handling processes which are compliant to the ISO15189 standard and FSR codes and training the relevant personnel in this process, in addition to changing their consumables to the required forensic DNA grade products.
Next, using the data provided in the SARC survey, ~5 consumables from each SARC were selected, ensuring that each SARC stocked the consumables chosen and that overall we would be testing a representative number of each consumable type. SARCs were sent instructions and evidence bags to package the required consumables and these were collected by a courier service and delivered to Cellmark Forensic Services.
Cellmark Forensic Services tested 261 forensic DNA grade consumables and every one of these consumables passed testing, as per the acceptance criteria for DNA consumables defined in FSR-C-108.
The results from this National Consumable Validation have demonstrated that the above end to end process is fit for purpose and at each stage of this process DNA contamination of the consumable has not occurred. However the evaluation of the processes that occur during the forensic medical examination have not been included in this validation and will require individual assessment by each SARC to demonstrate that their end to end process is fit for purpose.
In addition, 14 non forensic DNA grade consumables were tested and 6 of these items failed, whereby unacceptable levels of DNA were identified on these consumables, further demonstrating the importance of SARCs using forensic DNA grade consumables for the recovery of forensic evidence.
The results have been collated and a National Consumable Validation Report has been developed and issued- available on the Knowledge Hub.
In addition, the FCN are proposing to the FSR that the data from the National Consumable Validation provides sufficient evidence to demonstrate that where a manufacturer has robust DNA-free treatment procedures in place, including ethylene oxide treatment and QC testing to demonstrate effectiveness, that forensic DNA grade consumables are fit for purpose and therefore there is no further requirement for forensic services to conduct routine batch testing on forensic DNA grade consumables, unless there is a significant change to the manufacturing of the item.